OphteisBio 1.6%
Cohesive - Designed for all types of surgery
- Optimal maintenance of volume in the anterior chamber or capsular bag
- Good coating power
- Easy injection and removal during surgical stages
OphteisBio 1.8%
Cohesive - Designed for small incision
- Good cohesivity at low shear-rate for a stable anterior chamber
- Strong coating of tissue thanks to improved dispersive property
- Easy to aspirate with high molecular weight
OphteisBio 3.0%
Dispersive - Designed for excellent endothelial protection
- Low molecular weight, high NaHA concentration
- Assures maximum protection and viscosity
- Good maintenance of anterior chamber
| Product | OphteisBio 1.6% | OphteisBio 1.8% | OphteisBio 3.0% |
| Polymer Origin | Biofermentation | Biofermentation | Biofermentation |
| Sodium Hyaluronate Concentration | 1.6% | 1.8% | 3.0% |
| Molecular Weight (Dalton) | approx. 3 million | approx. 3 million | approx. 0.75 million |
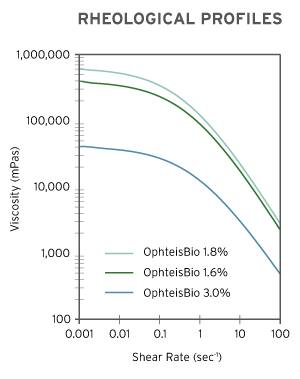
| Zero Shear Viscosity (mPas) | avg. 400,000 | avg. 600,000 | avg. 30,000 |
| Osmolality (mOsm/kg) | 300 to 350 | 300 to 350 | 300 to 350 |
| pH | 6.8 - 7.6 | 6.8 - 7.6 | 6.8 - 7.6 |
| Shelf life (years) | 3 | 3 | 3 |
| Storage | 2°C to 25°C | 2°C to 25°C | 2°C to 25°C |
| Syringe volume (ml) | 1.1 | 1.1 | 1.1 |
| Cannula (gauge) | 27G | 27G | 25G |

| Product name | OphteisBIO 1.6 |
| Product Code |
R-OPB16 |
| Presentation | Sterile single-use syringe with 1.1 ml 1.6% sodium hyaluronate solution and single-use 27g injection cannula, Luer-Lock |
| Product name | OphteisBIO 1.8 |
| Product Code |
R-OPB18 |
| Presentation | Sterile single-use syringe with 1.1 ml 1.8% sodium hyaluronate solution and single-use 27g injection cannula, Luer-Lock |
| Product name | OphteisBIO 3.0 |
| Product Code |
R-OPB30 |
| Presentation | Sterile single-use syringe with 1.1 ml 3.0% sodium hyaluronate solution and single-use 25g injection cannula, Luer-Lock |
Not all products or offerings are approved or offered in every market and approved labelling and instructions may vary from one country to another. For country specific product information contact your local distributor or email iol_enquires@rayner.com.
This product is not approved by the FDA for use or distribution in the United States of America.
Rayner hold a selection of EC certificates for various products. Please contact the Regulatory Affairs team for the current version.